Separation of Refrigerant R-410A Using Porous Materials: Molecular Insight into Binary Sorption of HFC-32 (difluoromethane) and HFC-125 (pentafluoroethane) Using Thermodynamic Measurements and Modeling
Jun 6, 2022 9:20am ‐ Jun 6, 2022 9:30am
Identification: 3727938
Global climate change is among the most prevalent issues in the 21st century. The United Nations (UN) published a report in 2021 projecting a global temperature increase of 1.5-2.0 K if anthropogenic greenhouse gas (GHG) emissions are not mitigated. This would lead to an increase in extreme weather patterns including hurricanes, droughts, heat waves, and flooding. Hydrofluorocarbons (HFCs) are among the most prevalent fluorochemicals and are used in applications including refrigeration and fire suppression. Due to large global warming potentials (GWPs), HFCs are currently being regulated by legislation including the Kyoto Protocol (2005), F-gas regulations in the European Union (2014), and the U.S. AIM Act (2020). As the production and use of HFCs is further restricted, responsible actions must be implemented to deal with the high-GWP HFCs that are currently being replaced by the next-generation refrigerants, hydrofluoroolefins (HFOs) and HFO/HFC blends.
Ideally, unused HFCs can be reclaimed, recycled, and repurposed into next-generation refrigerants or high-value feedstock. Since many HFCs are azeotropic or near-azeotropic mixtures, these refrigerants must first be separated so that recycling is made possible. This presentation will discuss the use of porous materials, including zeolites and activated carbons, for the separation of refrigerant R-410A (50/50 wt% HFC-32/HFC-125). Both pure gas and mixed gas sorption isotherms for HFC-32 and HFC-125 have been measured using Hiden Isochema XEMIS gravimetric microbalances for various sorbents including basic zeolites, acidic zeolites, high-silica zeolites, and activated carbons. Results will be analyzed and compared to elucidate molecular-level interactions and to assess separation capabilities for R-410A. Thermodynamic modeling of binary sorption using both Ideal Adsorbed Solution Theory (IAST) and Real Adsorbed Solution Theory (RAST) has been performed. Differences between model predictions will be compared and discussed to further investigate molecular-level sorption behavior and the prediction of large-scale separation processes.
Ideally, unused HFCs can be reclaimed, recycled, and repurposed into next-generation refrigerants or high-value feedstock. Since many HFCs are azeotropic or near-azeotropic mixtures, these refrigerants must first be separated so that recycling is made possible. This presentation will discuss the use of porous materials, including zeolites and activated carbons, for the separation of refrigerant R-410A (50/50 wt% HFC-32/HFC-125). Both pure gas and mixed gas sorption isotherms for HFC-32 and HFC-125 have been measured using Hiden Isochema XEMIS gravimetric microbalances for various sorbents including basic zeolites, acidic zeolites, high-silica zeolites, and activated carbons. Results will be analyzed and compared to elucidate molecular-level interactions and to assess separation capabilities for R-410A. Thermodynamic modeling of binary sorption using both Ideal Adsorbed Solution Theory (IAST) and Real Adsorbed Solution Theory (RAST) has been performed. Differences between model predictions will be compared and discussed to further investigate molecular-level sorption behavior and the prediction of large-scale separation processes.
A systems thinking approach to high school chemistry instruction through green chemistry principles
Jun 6, 2022 9:30am ‐ Jun 6, 2022 9:40am
Identification: 3728177
Three of the 12 Green Chemistry Principles can be readily taught to high school chemistry students and applied to their practical laboratory work, providing an holisitic means for students to measure experimental success, in addition to traditional reductionist success measures as experimental yield. This presentation outlines teaching and learning methods for introducing Green Chemistry Principles to high school students, through a student-directed, inquiry-based critical analysis of how experimental “success” is measured. Current and ongoing research into the impact of such learning on students' attitudes towards the subject of chemistry will be addressed.
Reactivity-directed analysis: A novel approach for the identification of toxic organic chemicals formed during water treatment with chemical oxidants
Jun 6, 2022 9:30am ‐ Jun 6, 2022 9:50am
Identification: 3728146
Treatment technologies that utilize chemical oxidants such as hydroxyl radicals, ozone, and chlorine are now widespread in a variety of water treatment applications (e.g., wastewater, water reuse, and drinking water). While these technologies are highly effective in removing organic and microbial contaminants, there is increasing evidence that their degradation can lead to the formation of a large variety of transformation products. The same is true for the reaction of chemical oxidants with dissolved organic matter (DOM), which is typically present in concentrations that are several orders of magnitude higher than those of anthropogenic chemicals. However, assessing the potential health risks that arise from the exposure to these complex chemical mixtures remains very challenging. One of the main reasons is the unavailability of approaches that enable an overall toxicity assessment of these mixtures while simultaneously providing information about the responsible toxicants’ identities. To address this issue, we developed a novel analytical approach called reactivity-directed analysis (RDA), which focuses on the detection and identification of organic electrophiles, the largest class of known toxicants. RDA utilizes the molecular toxicity mechanism of organic electrophiles, their reactivity with nucleophilic biomolecules such as proteins, to identify this class of contaminants in oxidative water treatment systems. This talk will discuss the results of laboratory experiments with individual organic contaminants and outline how RDA can be used as a novel tool for the identification of reactive organic electrophiles in complex environmental mixtures.
Online monitoring of small volume reactions using compact liquid chromatography instrumentation
Jun 6, 2022 9:30am ‐ Jun 6, 2022 9:50am
Identification: 3728044
Compact high performance liquid chromatography (HPLC) is an important step forward in reducing the environmental impact of this widely used analysis technique. The environmental benefits of compact HPLC include dramatically reduced footprint, weight, solvent consumption and waste, and lower cost. When combined with reaction monitoring, compact HPLC enables chemists and engineers to monitor the progress of chemical reactions as needed without consuming large amounts of solvent and generating excessive waste. Further, compact, capillary based HPLC systems can be used to sample from very small reaction volumes thus supporting chemical development approaches with less environmental impact while not affecting the reaction equilibrium. These concepts will be demonstrated using a commercial compact HPLC for online monitoring of as small as a 1 mL volume of chemical reaction, with automated sampling of nL-scale volumes directly from the reaction vessel. Monitoring short term and long term reactions were conducted using tandem on-capillary UV absorbance followed by in-line MS detection.
Importance of safer new chemicals screening tools to navigate smoothly through a new chemicals application
Jun 6, 2022 9:30am ‐ Jun 6, 2022 9:50am
Identification: 3728632
Since passage of the Frank R. Lautenberg Chemical Safety for the 21 Century Act in 2016, U.S. EPA has assessed more than 3,500 new chemical cases with the goal of determining whether a new chemical presents an unreasonable risk to human health or the environment. Submitting notice under §5 of TSCA (such as a premanufacture notice (PMN), Low Volume Exemption (LVE), Low Release, Low Exposure Exemption (LoREX), Test Marketing Exemption Application (TMEA) or Significant New Use Notice (SNUN)) to the Agency requires an applicant to expend significant amounts of time and resources testing and assessing exposure. Although the Agency has determined that only zero of more than 3,500 chemical cases submitted since 2016 present an unreasonable risk, not all new chemical applications are successful. In fact, more than 10% of new chemical applications are not successful for the applicant and do not result in a finding that the chemical is not likely to present an unreasonable risk.
In order to maximize the likelihood that the Agency will make an affirmative finding that a new chemical substance will not present an unreasonable risk to health or the environment, it is critical that all new chemicals undergo rigorous assessment using EPA's predictive tools and models. When used correctly, these tools and models can estimate a new chemical’s release to the environment, predict environmental fate, and assess the ability of a new chemical to expose workers and the general population and hazards. This presentation will identify many of the ideal characteristics of a low concern substance and will demonstrate the application and interpretation of U.S. EPA’s predictive tools.
In order to maximize the likelihood that the Agency will make an affirmative finding that a new chemical substance will not present an unreasonable risk to health or the environment, it is critical that all new chemicals undergo rigorous assessment using EPA's predictive tools and models. When used correctly, these tools and models can estimate a new chemical’s release to the environment, predict environmental fate, and assess the ability of a new chemical to expose workers and the general population and hazards. This presentation will identify many of the ideal characteristics of a low concern substance and will demonstrate the application and interpretation of U.S. EPA’s predictive tools.
Development of Data Science Tools for Catalyst Development
Jun 6, 2022 9:30am ‐ Jun 6, 2022 10:10am
Identification: 3726187
Chemists are often guided by intuition when predicting the how and where an organic molecule will react This is based on wisdom and a fundamental understanding of reactivity. However, while this tactic facilitates the majority of reaction development in organic and related chemical fields, the reality is that we rely significantly on empirically optimizing reactions and structures for a desired outcome and/or function. While a routine process, optimization protocols can cause a considerable investment of time and do not always provide an adequate solution. Therefore, we have aimed to develop a program that assists the rapid analysis of structure function relationships to reveal not only better systems, but also the underlying reasons for improved performance of substrates, catalysts, or other functional materials. This lecture will outline how we have put into practice a workflow that integrates data science tools, physical organic chemistry, and reaction optimization with a focus on the case study of the use of phosphines in a wide range of reactions.
Synthesis and characterization of semi-aromatic biobased polyesters exhibiting diverse mechanical properties
Jun 6, 2022 9:30am ‐ Jun 6, 2022 9:50am
Identification: 3727852
In this study, we describe a series of semi-aromatic polyesters prepared from coumaric, ferulic and sinapic acids that exhibit diverse mechanical properties. AB monomers were synthesized through the reaction of the phenolic alcohol with cyclic carbonates. Each of these starting materials can be derived from lignin and other biosources. The total synthesis was evaluated using Ecofactor and E-scale which suggests that these reactions are exceptionally eco-friendly. Polycondensation of these monomers yielded semicrystalline and amorphous polymers with a diverse range of properties including ductile thermoplastics, strong and rigid thermoplastics, and a series of thermoplastic polyester elastomers with shape memory, of which there are few biobased examples reported. The mechanical properties of the polymers varied, with strain at break ranging from 0.6% to 950% and Young’s moduli ranging from 2.8 MPa to 2400 MPa. Subsequent composting respirometry studies were performed on a several of these polyesters showing both degradable and nondegradable polymers that may provide a guideline for backbone architectures designed for compostability.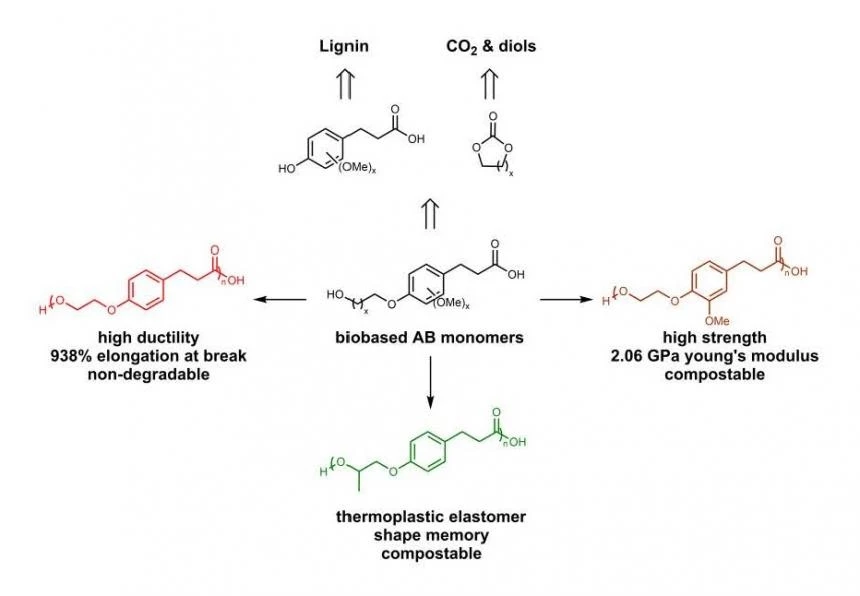

Lignin-based semi-aromatic polyesters offer diverse properties based on the synthesis of the biobased AB monomer.
Permeability, Solubility, and Diffusivity of HFC-32 and HFC-125 in perfluoro(butenyl vinyl ether) and perfluoro(2,2-dimethyl-1,3-dioxole) copolymers
Jun 6, 2022 9:30am ‐ Jun 6, 2022 9:40am
Identification: 3727956
Recent legislation such as the 2020 AIMS Act has called for increased regulation and eventual phaseout of hydrofluorocarbon (HFC) refrigerants that contribute to global warming. There are currently millions of kilograms of HFCs and HFC mixtures in global circulation. While many of these refrigerants exhibit high global warming potentials (GWP), some HFCs have lower GWPs and have the potential to be recycled and repurposed into next generation refrigerant mixtures. HFC mixtures are often azeotropic, thus making their separation difficult and energy intensive. With national and global efforts dedicated towards reducing the use of HFC refrigerants, sustainable methods that allow for the separation of HFCs are imperative. Membranes provide an attractive method for the separation of HFCs due to low energy consumption and lower capital requirements compared to other separation technologies. In order to investigate the use of membranes for HFC separation, the permeability, solubility, and diffusivity of difluoromethane (HFC-32, CH2F2) and pentafluoroethane (HFC-125, CHF2CF3), which are components of the widely used refrigerant R-410A, were measured in amorphous copolymers of perfluoro(butenyl vinyl ether) (PBVE) and perfluoro(2,2-dimethyl-1,3-dioxole) (PDD) at a range of compositions. Diffusivity and solubility results indicate that the separation of HFC-125 and HFC-32 through the amorphous polymers is predominately diffusivity driven. Ideal selectivity results indicate that a highly selective separation can be achieved with a copolymer containing a PDD composition near 80 mol%.
Separation of Azeotropic Refrigerant Mixtures using Extractive Distillation with Ionic Liquid Entrainers
Jun 6, 2022 9:40am ‐ Jun 6, 2022 9:50am
Identification: 3728467
Hydrofluorocarbons (HFCs) have been used as refrigerants globally since the 1990’s and replaced chlorofluorocarbons (CFCs), which were linked to the depletion of the Earth’s ozone layer. HFC refrigerants are being phased out over the next two decades due to their high global warming potential. The refrigerant industry is moving toward the production and marketing of HFOs and HFO/HFC refrigerant blends. This will require the recycling and repurposing of HFC mixtures, but many of the HFC mixtures are azeotropic or near azeotropic (i.e., R-410A, R-404A, R-407C, to name a few) making the separation of the components impossible using current methods. Extractive distillation using an ionic liquid entrainers offers a solution.
Ionic liquids (ILs) are being developed as novel entrainers for extractive distillation because of their negligible vapor pressure, thermal and chemical stability, and high and differential solubility with HFC refrigerants. Vapor liquid equilibria data for refrigerants difluoromethane (HFC-32), chlorodifluoromethane (HCFC-22), pentafluoroethane (HFC-125), 1,1,1-trifluoroethane (HFC-143a), and 1,1,1,2-tetrafluoroethane (HFC-134a) in ionic liquids 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)- imide ([C2C1im][Tf2N]) and 1-butyl-3-methylimidazolium hexafluorophosphate ([C4C1im][PF6]) were fit with the Peng−Robinson equation of state to simulate the separation of multi-component azeotropic refrigerant mixtures and to develop rate-based and equilibrium models in ASPEN Plus. Process flow diagrams were developed and optimized based on a set of physical and chemical constraints with the goal to optimize parameters and achieve refrigerant grade (>99.5 wt %) purity. The ionic liquids were found to be effective entrainers for separating refrigerant mixtures.
Ionic liquids (ILs) are being developed as novel entrainers for extractive distillation because of their negligible vapor pressure, thermal and chemical stability, and high and differential solubility with HFC refrigerants. Vapor liquid equilibria data for refrigerants difluoromethane (HFC-32), chlorodifluoromethane (HCFC-22), pentafluoroethane (HFC-125), 1,1,1-trifluoroethane (HFC-143a), and 1,1,1,2-tetrafluoroethane (HFC-134a) in ionic liquids 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)- imide ([C2C1im][Tf2N]) and 1-butyl-3-methylimidazolium hexafluorophosphate ([C4C1im][PF6]) were fit with the Peng−Robinson equation of state to simulate the separation of multi-component azeotropic refrigerant mixtures and to develop rate-based and equilibrium models in ASPEN Plus. Process flow diagrams were developed and optimized based on a set of physical and chemical constraints with the goal to optimize parameters and achieve refrigerant grade (>99.5 wt %) purity. The ionic liquids were found to be effective entrainers for separating refrigerant mixtures.
